CINE study brings new insight in the selection of the best salts for electrolytes in lithium-oxygen batteries.
With an economy less based on fossil fuels and more supported on electricity, the leading role of batteries, supercapacitors and other energy storage devices increases. And grows the need to improve these electrochemical devices in terms of cost and performance, as well as to adapt them to the needs of different applications– namely of electric cars and electronic devices.
One of the promising technologies to this effect is that of lithium-oxygen batteries, whose operation is based on lithium oxidation in the anode and oxygen reduction in the cathode. Capable of supplying energy density ten times greater than traditional lithium-ion batteries, they are particularly interesting for large scale storage of solar or eolian energy.
A research conducted by researchers from CINE’s Advanced Energy Storage Division, in collaboration with scientists from Notre Dame University (USA), discussed one of the main challenges to the effect of improving the performance of lithium-oxygen batteries: the right selection of salts and solvents which make up the electrolyte. In contact with the positive and negative electrodes, the electrolyte is the means responsible for triggering the reactions of oxidation and reduction of and for transporting the positive ions during battery charge and discharge processes.
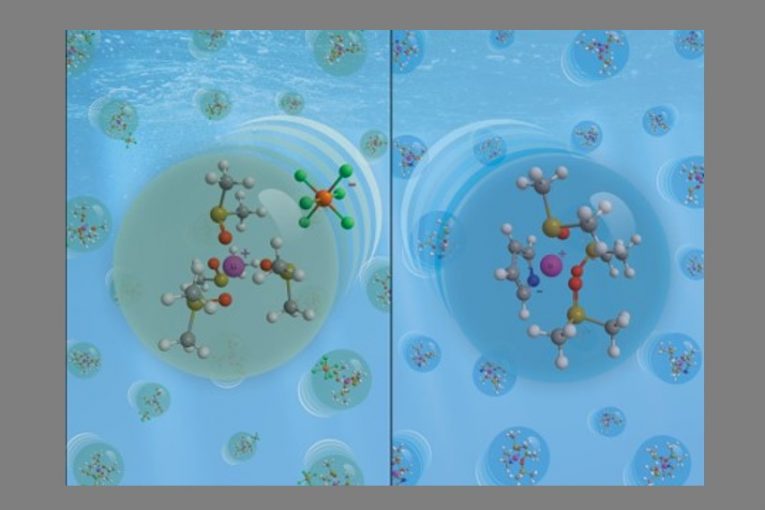
CINE’s team focused on the study of two solutions which can be used as electrolytes, composed of the solvent DMSO and two different lithium salts. The objective was to understand in detail certain very significant properties in the battery’s performance.
The research was conducted using computational methods (molecular dynamics simulation). “In these simulations, we used standards already defined by the literature, but also created post-processing codes to calculate certain properties such as solvation and ionic conductivity structures more accurately”, says professor Gustavo Doubek (UNICAMP, Brazil), who coordinated the work.
While ionic conductivity is related to the electrolyte’s ability to conduct the lithium ions in the battery, solvation refers to the chemical reactions which occur between the solvent and the solute. “Solvation informs us how the lithium ions become chemically ‘protected’ by the solvent’s molecules”, explains Doubek, who adds that this condition influences the reactions which occur on the lithium-ion surface when they come in contact with the oxygen in the cathode. “Such information is very difficult to obtain experimentally; thus, simulation helps a lot in this determination”, he completes.
The simulations were made in the context of the PhD of Juliane Fiates, with the supervision of Doubek. The paper was started at the Chemical Engineering College of UNICAMP and was finalized at Notre Dame University, during a research internship taken by the PhD student.
The research was highlighted in one of the covers of the Physical Chemistry Chemical Physics.
Paper: Impact of anion shape on Li+ solvation and on transport properties for lithium-air batteries: a molecular dynamics study. Juliane Fiatesa, Yong Zhang, Luís F. M. Franco, Edward J. Maginn and Gustavo Doubek. Phys. Chem. Chem. Phys., 2020,22, 15842-15852. https://doi.org/10.1039/D0CP00853B.











