Methane to Products
Principal Investigator (PI)
Fabio Coral Fonseca – IPEN – fabiocf@usp.br
Co-PIs
Almir Oliveira Neto – IPEN – aolivei@ipen.br
André Santarosa Ferlauto – UFABC – andre.ferlauto@ufabc.edu.br
Daniel Zanetti de Florio – UFABC – daniel.florio@ufabc.edu.br
Elisabete Inacio Santiago – IPEN – eisantia@ipen.br
Estevam V. Spinacé – IPEN – espinace@ipen.br
Reginaldo Muccillo – IPEN – muccillo@usp.br
________
Publications
See publications list.
________
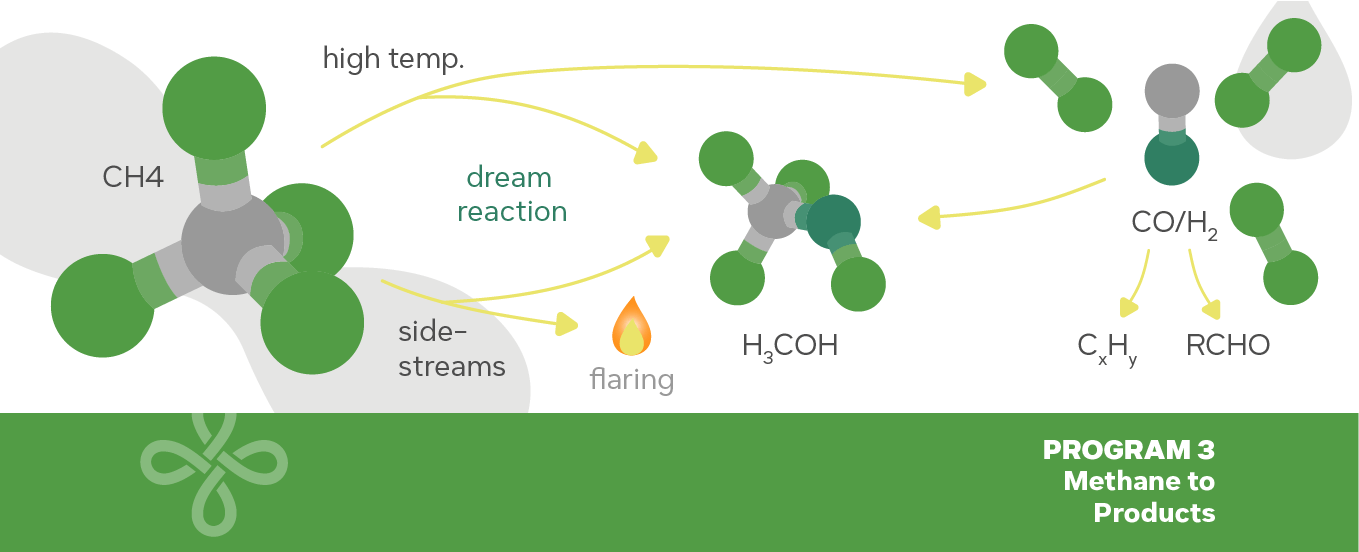
Amongst the challenges facing the modern chemical industry, the development of new, sustainable routes for production of chemicals and fuels is potentially the most significant. Natural gas has been and will continue to be, in the near future, one of the main sources of hydrocarbons for energy generation. Natural gas, with its major component being CH4 , has become significantly cheaper and with availability well beyond the current and future predicted demand. Such a scenario has renewed scientific interest in technologies that can efficiently and sustainably convert such abundant feedstock into useful products. Nevertheless, methane is also the least reactive of all hydrocarbons and, therefore, development of efficient and selective processes of methane conversion to fuels and chemicals has become critical in recent years due to the emerging number of untapped natural gas reserves and the urgent need to reduce both greenhouse gas emissions and dependence from limited crude oil resources. However, industrial routes for one-step conversion of methane to fuels and chemicals are scarce. The most technologically advanced routes involve the indirect conversion of methane (i.e., methane steam reforming) to fuels and chemicals. The challenges associated with the one-step conversion of methane to chemicals and fuels arise from the fact that methane is a very stable molecule, comprised of C-H bonds that are weakly polarized. Hence, very aggressive reactants and operating conditions (e.g., temperature and pressures) are required to activate the methane C-H bond, consequently favoring undesired reactions (e.g., complete combustion to CO2 ) which lead to loss in activity, selectivity, and yield of desired products.
________
Projects
Co-PI: Reginaldo Muccillo – IPEN – muccillo@usp.br
The objective of the project is the development of a new generation of composite membranes for the separation of CO2 with increased electrical conductivities. To achieve this goal, the ionic and electronic conductivities will be manipulated by the microstructure or composition.
Co-PI: Estevam V. Spinacé – IPEN – espinace@ipen.br
The main objective of this proposal is to prepare active photocatalysts with different compositions and morphologies based on noble metal nanoparticles (Pt, Au, Pd, Ag) supported on semiconductors (TiO2, ZnO, Ga2O3 ) for the conversion of methane to high added value products (C2 to C6 hydrocarbons, especially C2H6 ethane) coupled to the evolution of hydrogen from water. The final objective is to obtain photocatalysts that present values of quantum efficiencies higher than those currently described in the literature (of the order of 5%).
Fabio Coral Fonseca – IPEN – fabiocf@usp.br
André SantarosaFerlauto – UFABC – andre.ferlauto@ufabc.edu.brThe project aims to develop new composites based on ceria for efficient thermochemical conversion of methane and other molecules at high temperature (up to 1500 K). The main goal is to obtain materials that are physically and chemically stable and that maintain high porosity in the long term, that is, that maintain catalytic activity under the operating conditions of the thermochemical redox cycles.
Almir Oliveira Neto – IPEN – aolivei@ipen.br
Thiago Lopes – IPEN – thiago.lopes@ipen.brThe project intends to promote the selective oxidation of methane to methanol by electrochemical means in a simple step through the development of electrocatalysts: (i) selective for the formation of hydrogen peroxide in–situ; (ii) capable of replicating the oxidation cycle of methane to methanol present in metalloenzymes; and (ii) based on conventional heterogeneous catalysis, but operating properly under an electrochemical control at intermediate temperatures.
Co–PI: Elisabete Inacio Santiago – IPEN – eisantia@ipen.br
The project aims to develop new electrode and electrolyte materials to be used in a methane (CH4) to methanol (CH3OH) electrochemical reactor. In this context, it is intended to produce polymeric solid electrolytes carrying carbonate ions (CO32–) with high ionic conductivity, low gas permeability and high chemical and mechanical stabilities, as well as selective and efficient electrocatalysts for both anode and cathode electrodes. The materials with the best physico–chemical properties will be evaluated in an electrochemical reactor capable of producing methanol from the methane (CH4) mediated by carbon dioxide (CO2) at low temperatures (<80 °C).
Fabio Coral Fonseca – IPEN – fabiocf@usp.br
Daniel Zanetti de Florio – UFABC – daniel.florio@ufabc.edu.brThe main goal is to develop a stable and high–performance solid oxide reactor to study electrolytic and galvanic reactions of methane at high temperatures. Such reactions will be used to obtain high value products such as methanol. The main challenge is to control the reaction paths and the stability of the system in both modes of operation, such as a Solid Oxide Fuel Cell (SOFC) and as a Solid Oxide Electrolyzer Cell (SOEC). The main expected result is the advance in the understanding of the main reactions that occur in galvanic reactors and the demonstration of the possibility of production of high value–added chemicals from methane in SOEC / SOFC reactors.










