Additive studied at CINE improves electrolyte properties for lithium-metal batteries
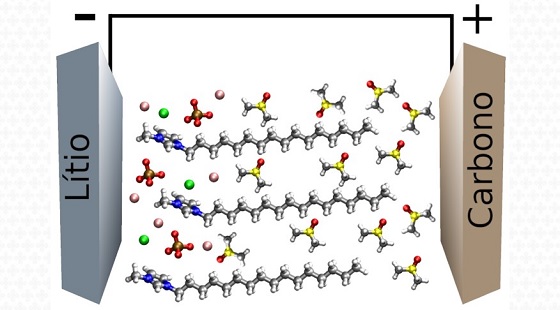
A recent CINE study showed that the addition of an ionic liquid crystal to a solvent widely used as electrolyte in lithium-metal batteries makes the compound more chemically stable and improves its ionic conductivity. With these properties, the electrolyte additive shows promise to produce more durable, safer and better performing batteries.
Developing more stable electrolytes, in fact, is one of the great challenges of battery research, especially concerning the promising lithium-metal batteries. This emerging technology, which includes lithium-oxygen batteries, is capable of vastly surpassing lithium-ion technology, the most used today, in terms of the amount of energy it can store. However, it comes up against the instability of the electrolyte.
The electrolyte of a battery is the component that is in contact with the two electrodes. Often, as in the case of this study, it is a liquid solution formed by a solvent and salts. Its main function is to actively provide the path for ions to move between electrodes carrying electrical charges – a process essential to battery charging and discharging. Therefore, battery electrolytes need to be good ionic conductors as well as chemically stable.
In fact, poor chemical stability makes the electrolyte more susceptible to forming unwanted, and sometimes toxic, compounds that can damage other battery components, leak, or cause the battery to heat up or explode. “An example of electrolytes that form unwanted components is when we observe a battery with a certain time of use and it appears to be dilated or swollen”, illustrates Tuanan Lourenço, first author of the article which reports on CINE’s work in the Journal of Materials Chemistry A.
The research brought together nine members from two CINE research divisions, the Computational Materials Science and Chemistry (CMSC) and the Advanced Energy Storage (AES) . “As the teams have different backgrounds and experiences, the meetings were very fruitful; each one was able to interpret the data from different and complementary points of view”, says Tuanan. According to him, the discussions generated innovative ideas, both for designing new experiments or calculations and for interpreting the data already obtained.
In the paper, the authors report the investigation of the properties of an electrolyte based on the organic solvent DMSO, often used in lithium-metal batteries, enriched with an ionic liquid crystal based on the organic imidazole compound. Ionic liquid crystals are salts that resemble ionic liquids in that they are liquid at room temperature, but differ from them in that they exhibit solid crystalline phases.
Using computational tools and experimental techniques, the authors studied in detail the effects of ionic liquid crystal addition on the physical, electrochemical and structural properties of the electrolyte and were able to establish what is the optimal ratio of additive to solvent.
“With the experiments we observe what is happening with the system in a macroscopic way, we obtain the measurements of the properties for matter as it is, since we are not using any model to represent the system”, explains Tuanan, who is a postdoctoral fellow at CMSC – CINE. “With computer simulations, we can obtain more detailed information from an atomistic point of view, observing how the atoms are organized, how they are interacting”, he adds.
The work showed that the addition of the ionic liquid crystal to a certain extent increases both the chemical stability and the conductivity of the electrolyte. “In practical terms, if we have a higher chemical stability and conductivity, we have an electrolyte with a better performance, and we can perhaps have a battery with a better performance”, summarizes Tuanan.
Tests of the electrolyte inside a battery were not reported in this article, which focused on the properties of the compound, but have already been performed. In fact, the real performance of a lithium metal battery with electrolyte additive with ionic liquid crystal was described in a patent authored by researchers from AES – CINE, which was licensed by Shell.
The research received financial support from FAPESP and Shell, in addition to strategic support from ANP and computational resources from USP and LNCC.
Paper reference: Tuning aprotic solvent properties with long alkyl chain ionic liquid for lithium-based electrolytes. Tuanan C. Lourenço, Letícia M. S. Barros, Chayene G. Anchieta, Thayane C. M. Nepel, Júlia P. O. Júlio, Luis Gustavo Dias, Rubens Maciel Filho, Gustavo Doubek and Juarez L. F. Da Silva. J. Mater. Chem. A, 2022.10, 11684-11701. https://pubs.rsc.org/en/content/articlelanding/2022/TA/D1TA10592B
Authors of the article who are members of CINE: Tuanan C. Lourenço (post-doc), Letícia M. S. Barros (master’s student), Chayene G. Anchieta (collaborating researcher), Thayane C. M. Nepel (post-doc), Júlia P. O. Júlio (master’s student) , Luis Gustavo Dias (professor at USP), Rubens Maciel Filho (professor at UNICAMP), Gustavo Doubek (professor at UNICAMP) and Juarez L. F. Da Silva (professor at IQSC-USP).
Contact

Juarez L. F. Da Silva
IQSC-USP











