CINE research contributes to the generation of electricity from ethanol using fuel cells.
Brazil is the second world producer of ethanol and the first in sugar cane ethanol. This renewable fuel is widely used in the country, where it is sold in all gasoline stations. The novelty is that ethanol can not only fill the tanks of internal combustion engines cars, but also power electric cars.
In fact, ethanol can be used to generate hydrogen and, from it, to produce electricity. The process, which is neutral in carbon emissions, is fully performed in a single device: a solid oxide fuel cell (SOFC).
In the electric car powered by ethanol, whose first prototype was launched by Nissan in 2016, there are no hydrogen tanks and the batteries do not require switches to recharge. Instead, there is a cell and ethanol fuel.
Now, a research led by the principal investigator of the CINE division “Methane to Products”, Fábio Fonseca, gave a very important step in the direction of improving the performance of these fuel cells. “This study goes deeper in a sequence of studies in which we try to advance the use of ethanol in fuels cells of solid oxides”, says Fonseca, who is a senior technologist of the Energy and Nuclear Research Institute (IPEN), where he also acts as manager of the Fuel and Hydrogen Cells Center.
“The impact that this technology may have in Brazil is huge”, comments Fonseca. “We can think of cars which do not require complex hydrogen tanks, and which can fuel in any station, charging as quick as filling the ethanol tank. We can go beyond and take electricity to communities far from the grid, just supplying them (if they have not already been supplied) with ethanol– a dense liquid energy carrier, renewable and available!”, he completes.
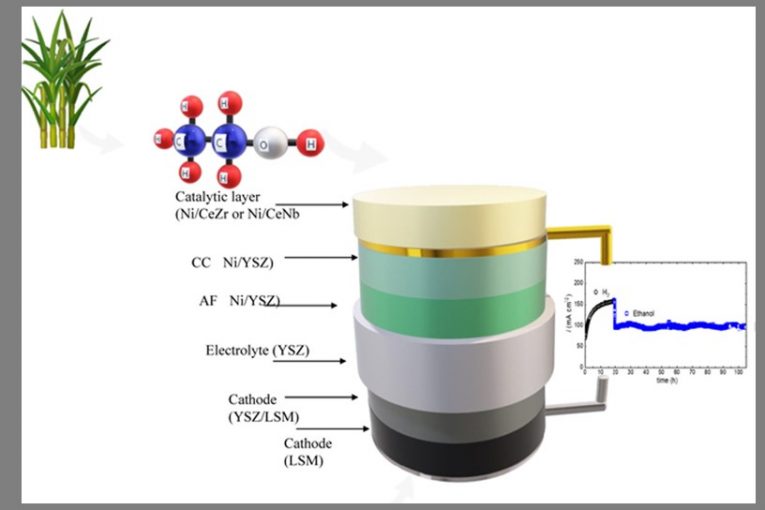
The SOFCs studied by Fonseca and his collaborators are formed by layers of different materials which perform complementary functions. Two layers make up the anode. In the catalyst layer, ethanol is transformed into hydrogen and compounds based on carbon. In the electrochemistry layer, the chemical energy from hydrogen is converted into electric energy by redox reactions. However, the process is still limited, especially concerning the formation of carbon deposits in the cell, which impair its performance in time.
Thinking about resolving this problem, Fonseca and his collaborators developed some variants of the material which makes up the catalyzing layer of anode, normally consisting of a nickel (Ni) and cerium oxide (CeO2) composite. The researchers introduced small portions of other elements (all non-precious metals) in the cerium oxide and assessed the performance of each new variant as a catalyst of ethanol conversion in SOFC. “We studied systematically the use of doping elements seeking to improve performance and minimize dependence on precious metals in the internal and direct conversion of ethanol into electricity”, says Fonseca. “The final idea is to have stability and avoid the degradation of the device”, he completes.
The study showed that cerium oxide doped with zirconium or niobium prevents carbon deposits without impairing the decomposition of ethanol in hydrogen and keeping the working of SOFC stable for at least 100 hours. In other words, the material proved efficiency to transform ethanol into hydrogen without generating undesirable effects in solid oxide fuel cells.
The research was a collaboration among Brazilian institutions IPEN, UFF, IME and INT, and the Université Grenoble Alpes (France.
The role of the ceria dopant on Ni / doped-ceria anodic layer cermets for direct ethanol solid oxide fuel cell. A.A.A.da Silva, M.C.Steil, F.N.Tabuti, R.C.Rabelo-Neto, F.B.Noronha, L.V.Mattos, F.C.Fonseca. Volume 46, Issue 5, 19 January 2021, Pages 4309-4328. https://doi.org/10.1016/j.ijhydene.2020.10.155
Contact

Fábio Fonseca
USP, Brasil











