CINE study advances the development of catalysts for lithium-oxygen batteries
CINE researchers and collaborators have taken an important step in the development of catalysts to optimize the performance of lithium-oxygen (Li-O2) batteries. These devices, which are still in the development stage, stand out for their ability to store much more energy than lithium-ion batteries, but they still face challenges in improving their cyclability (the amount of recharges the battery allows before end of life).
“This work contributes to the construction of a Li-O2 battery with better cycle efficiency and greater durability, without using noble or high-cost materials”, summarizes Professor Gustavo Doubek (UNICAMP), researcher in the Advanced Energy Storage division at CINE.
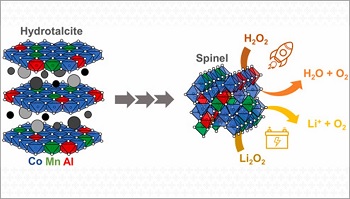
The operation of lithium-oxygen batteries is based on the reaction of lithium ions with oxygen from the air. In the battery discharge, step in which the device delivers energy in the form of electricity, this reaction generates lithium peroxide (Li2O2), among other compounds. In charge, when energy is stored, the products decompose.
The charge-discharge cycle is repeated and, for the battery to maintain good performance over many cycles, it is essential that all the formed lithium peroxide decomposes quickly. Therefore, in recent years, scientists in various parts of the world have dedicated efforts to develop and study catalysts which combine relatively low cost, good performance and good durability, to promote the decomposition of lithium peroxide,
Metal oxides of the spinel family based on cobalt (Co), manganese (Mn) and aluminum (Al) are among the most promising materials for this application. These catalysts can be produced using a simple, two main step method. Initially, the precipitation of a solution forms cobalt, manganese and aluminum oxides, mainly from the family of hydrocalcites – lamellar materials, similar to clays. In the second stage, which consists of a thermal treatment, that is, heating the precipitates to 900 ◦C, more compact metallic oxides, are obtained, called spinels.
This simple method had already been used to produce catalysts used to decompose Li2O2, as well as H2O2 (hydrogen peroxide) for application in satellite micropropulsion. However, the results were not always reproducible, generating catalysts of different qualities and performances. The problem caught the attention of CINE researchers, who were searching to optimize the cycling of lithium-oxygen batteries. They teamed up with collaborators from the Brazilian National Institute for Space Research (INPE) who were interested in the application of catalysts to satellites.
The team planned and carried out a series of experiments in order to understand which parameters of this method affect the final performance of the spinels as catalysts for the decomposition of peroxides. They changed, one by one, several synthesis conditions (pH and initial composition of the solution, aging and washing of the precipitates, temperature of the heat treatment) and analyzed the characteristics of each of the materials obtained, mainly their catalytic activity and their mechanical resistance.
The materials with the best characteristics were tested as catalysts for the decomposition of Li2O2 in a lithium-oxygen battery. For this, the scientists prepared a cathode based on carbon nanotubes “decorated” with spinel catalysts (particles of up to 200 nanometers). For comparison purposes, the researchers also produced a spinelless cathode. The cathode with catalysts was able to decompose almost all of the lithium peroxide and significantly improved battery performance, not only in charging but also, unexpectedly, in discharging.
The study reinforced the advantages of spinel as catalysts for lithium-oxygen battery cathodes, as well as the importance of controlling the parameters in the process to obtain these materials.
Scientific article reference: Conversion of Co-Mn-Al Hydrotalcites in Highly Active Spinel-type Catalysts for Peroxide Decomposition. André Navarro de Miranda, William Gonçalves Nunes, Leandro José Maschio, Luis Gustavo Ferroni Pereira, Sayuri Okamoto, Ricardo Vieira e Gustavo Doubek. Catalysis Today (in press). https://doi.org/10.1016/j.
Authors of the article who are CINE members: André Navarro de Miranda (doctoral student at UNICAMP), Willian Gonçalves Nunes (doctoral student at UNICAMP) and Gustavo Doubek (professor at UNICAMP and researcher at CINE).
Contact

Gustavo Doubek
UNICAMP - Brasil

